Ad5FGF-4
PROPOSED GENERX TREATMENT ALGORITHM
Based on FDA-Cleared U.S. Phase 3 Clinical Study Design
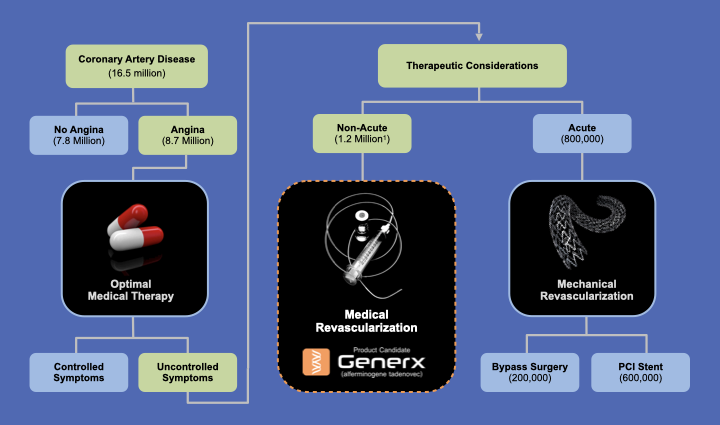
Patients with Refractory Angina who could potentially Benefit from Medical Revascularization:
(1) Are no longer responsive to optimal small molecule anti-anginal drug therapy
(2) Would not have a current or prophylactic benefit from mechanical revascularization procedures, that include percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG)
(3) Continue to experience refectory angina following a mechanical revascularization procedure.


Design of the FDA-cleared Phase 3 AFFIRM study is based on characteristics of patient responders in prior studies, as well as a fundamental research discovery relating to dramatic improvement in gene transfection in the heart following the induction of transient myocardial ischemia, and demonstration in a small international study that balloon catheter-based delivery of Generx under conditions of transient ischemia is safe (based on measurement of serum troponin levels, an indicator of damage to heart muscle).
