Cardiac Angiogenic Gene Therapy Finding New Ways To Heal
Main Content
Cardiac Angiogenic Gene Therapy: Finding New Ways to Heal
Gene Biotherapeutics is a publicly-traded, clinical development-stage biotechnology company engaged in the clinical advancement and commercialization of DNA-based biotherapeutics, primarily for the treatment of cardiovascular disease.
Clinical Trial
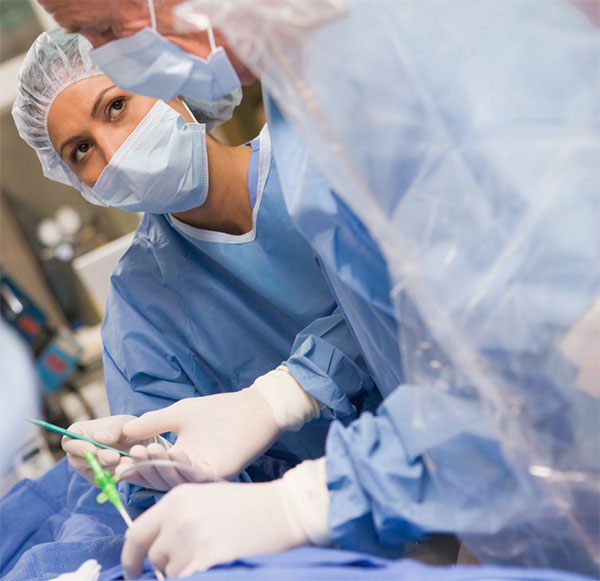
The Phase 3 “AFFIRM” clinical study evaluates Gene Biotherapeutics’ lead product candidate, Generx [Ad5FGF-4], as a one-time administration for the treatment of refractory angina due to late-stage coronary artery disease.
Our Science
Gene Biotherapeutics’ angiogenic gene therapy is designed to improve cardiac perfusion (blood flow) and to increase the supply of oxygenated blood by promoting the growth of microvasculature in ischemic cardiac tissue.
Generx Product Candidate
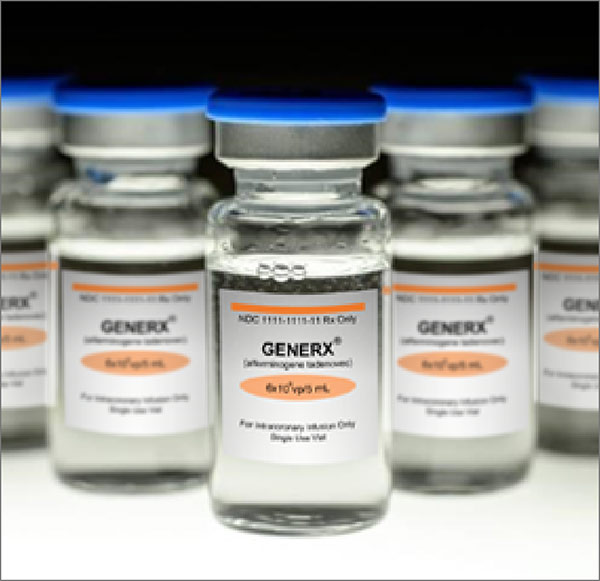
Generx [Ad5FGF-4] is engineered to express secreted human fibroblast growth factor-4 (FGF-4) protein, a key regulator of angiogenesis. Generx is administered by an interventional cardiologist during a brief procedure similar to a diagnostic angiogram.
Take a moment to answer these few simple questions to see if you're a candidate for the clinical study.


